Fifteen subjects with bronchial asthma were initially enrolled in the study, and selected according to the following criteria: age between 20 and 30, in an asthma-free interval, (ie, no attack of asthma in the last four weeks), clear cut history of attacks of asthma triggered by a well defined environmental allergen, and positive skin (prick) and radio-allergosorbent (RAST) tests to that allergen. Because 10 mg of intravenously administered chlorpheniramine dilates the bronchi only if FEV1 is low, the FEV1 of our subjects had to be higher than 60 percent of the predicted if a broncho-dilating effect of such an intravenous dose was to be avoided; the variability (ie, range/mean) of the three highest FVC and FEVj had to be less than 5 percent within the day and less than 10 percent from day to day. Nine out of these 15 subjects were finally included in the study: these nine all showed reproducible responses to both inhaled histamine and allergen. In these nine subjects, an identical dose of drug or allergen produced airway responses of comparable magnitude (—A FEV1 between 10 and 18 percent).
Histamine dihydrochloride (Calbiochem, Los Angeles), allergens (Hollister-Stier, Spokane*), and in three subjects, acetylcholine chloride (Calbiochem, Los Angeles) as well, were aerosolized in saline solution through a disposable manifold nebulizer (Bard-Parker, Rutherford, N.J.). The mouth piece was removed, and the subject was asked to breathe directly from the nebulizer with lips tightened around the exit port. The particle size distribution of a saline aerosol ranged between 0.8/u and 6.0^ for 80 percent of the particles (information provided by manufacturer and double-checked by Dr. Peter Lee from the Pulmonary Section of the Abraham Lincoln School of Medicine, University of Illinois, Chicago). The nebulizer was connected in line with a volume ventilator (Monaghan 225, Littleton, Colo). The tidal volume was set at 500 ml and the plateau flow at 2 L/second. The nebulizer was activated in inspiration only. The airflow powering the nebulizer was 6 L/min-ute. The fluid (saline) output was 0.10 ml/10 breaths. We calibrated the flow, volume, and duration of air delivered by the ventilator with a 13 L Collins spirometer and a computerized system with flow as primary signal. With the ventilator in assist mode, histamine, acetylcholine, and allergen were inhaled during a sequence of ten breaths. The dose delivered was expressed in milligrams base for drugs or in protein nitrogen units (PNU) for allegens.

Chlorpheniramine maleate in a dose of 10 mg, was injected slowly intravenously after test doses of 8 mg orally and 5 mg intravenously administered on separate days had failed to produce drowsiness, sleepiness, dryness of nasal or buccal mucosa, or accommodation changes. This screening procedure was prompted by the severe drowsiness experienced by one subject after intravenous administration of 10 mg. This subject was not included in the study. In the three subjects receiving atropine, we planned to use progressively increasing doses, starting with 0.4 mg given subcutaneously. The ECC was monitored for one hour after the administration of chlorpheniramine or atropine.
Flow volume curves were recorded with a computerized system using flow as primary signal. Mean FVC, FEVj, peak expiratory flow (FEFmax) and forced expiratory flow at 50 percent, 75 percent, between 25 percent and 75 percent of the vital capacity (FEF50%, FEF755K and FEF25-75% respectively) were obtained from the three flow volume loops with the highest FEV1. A typical nebulization session consisted of the recording of flow volume curves before and after increasing concentrations of histamine, acetylcholine, or allergen until the mean FEVt decreased with at least 10 percent (threshold response), and the subjects complained of chest tightness, shortness of breath, or wheezing. Thus, all threshold responses in this study were characterized by a change in FEV1 of twice its spontaneous variability and by symptoms and signs consistent with acute airway constriction. All the mean A FEV1 preceding the threshold response were less than 5 percent of the baseline value.
Two main reasons have influenced the selection in our inhalation tests of an endpoint characterized by —A FEVj > 1056 rather than the more popular —A FEV1 > 20 percent: (a) The design of the study required the use of mild attacks of drug- or allergen-induced asthma. Histamine is only one of the mediators of IgE-induced allergic manifestations, and therefore, the dose ratio of chlorpheniramine against allergen should be smaller than the dose ratio of this H1 blocker against histamine. It follows that in a study concerned with the possible effects of chlorpheniramine against allergen-induced asthma, the administered dose of this drug should increase the tolerance to inhaled histamine at least fourfold. A ratio of chlorpheniramine against histamine lower than 4, eg, 2, might not reveal the protective effect of this H1 blocker against allergen induced asthma.  For attacks of asthma with a higher physiologic endpoint, eg —A FEVt > 20%, doses of chlorpheniramine higher than 10 mg intravenously would have been indicated in order to achieve a dose ratio of 4 against histamine. Such doses are potentially toxic and potentially bronchodilator. It is not clear why a certain dose of chlorpheniramine, eg, 10 mg intravenously, dilates the bronchi only if these are moderately or severely constricted. One explanation, tempting but not proven, is that moderately or severely constricted bronchi are surrounded by more histamine than the normal or mildly constricted ones. The bronchodilator effect would have made the protective effect of chlorpheniramine difficult to interpret. (b) During our preliminary tests with a different endpoint, PD20, two subjects challenged with inhaled allergen according to the recommended protocol developed biphasic attacks of asthma with the late component lasting several days. In our previous experience with approximately 1,500 inhalation tests with allergen, such an incident occurred only four other times. In spite of its rarity, considering the basic needs of the experimental protocol, the validity of —A FEV1 > 10 percent (see below) and the fact that these two incidents occurred within one year at the same institution, we elected to measure only small, “threshold” airway response.
For attacks of asthma with a higher physiologic endpoint, eg —A FEVt > 20%, doses of chlorpheniramine higher than 10 mg intravenously would have been indicated in order to achieve a dose ratio of 4 against histamine. Such doses are potentially toxic and potentially bronchodilator. It is not clear why a certain dose of chlorpheniramine, eg, 10 mg intravenously, dilates the bronchi only if these are moderately or severely constricted. One explanation, tempting but not proven, is that moderately or severely constricted bronchi are surrounded by more histamine than the normal or mildly constricted ones. The bronchodilator effect would have made the protective effect of chlorpheniramine difficult to interpret. (b) During our preliminary tests with a different endpoint, PD20, two subjects challenged with inhaled allergen according to the recommended protocol developed biphasic attacks of asthma with the late component lasting several days. In our previous experience with approximately 1,500 inhalation tests with allergen, such an incident occurred only four other times. In spite of its rarity, considering the basic needs of the experimental protocol, the validity of —A FEV1 > 10 percent (see below) and the fact that these two incidents occurred within one year at the same institution, we elected to measure only small, “threshold” airway response.
The use in inhalation tests of an endpoint characterized by —A FEVj > 10 percent was considered to be physiologically valid for the following reasons:
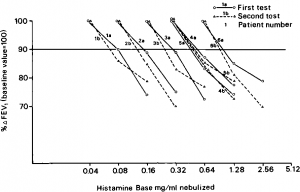
- As shown in Figure 1, in six asthmatic subjects we have obtained reproducible threshold doses using as physiologic endpoint —A FEVj > 10 percent This is not at all surprising since Tiffeneau, with an identical endpoint, has repeatedly reported that the inhalation tests with histamine, acetylcholine, and allergen were reproducible in most subjects.
- In antigen challenge tests, characterized by mild comparable airway responses and presence of wheezing, an increase in the concentration of allergen leads to an increase in the airway responses.
- Our arbitrary threshold response is not at variance with PD20, characterized by —A FEVj > 20 percent PD20 is calculated by the interpolation of two doses, one producing a FEVj response larger than —10 percent, but smaller than —20 percent and the other just above —20 percent It seems a reasonable assumption that if PD20 is valid, so are the lower (our threshold dose) and the higher —A FEVX used for its calculation. Then, a —A FEVi > 20 percent may not necessarily represent a larger airway response than —A FEVj > 10 percent if the variability of die former response is 10 percent and the variability of the latter response is only 5 percent This is consistent with a fundamental principle of statistical analysis: die significance of a change depends on both the magnitude of change and its variability.
- A physiologic endpoint similar to that adopted by us for this study has been extensively used by others. Tiffeneau’s findings in particular have been repeatedly confirmed by others, including the authors using PD20.» Incidentally, the provocation dose 35(—A Gaw/VL > 35 percent) proposed by Chai et al, and interchangeably used with PD20, is definitely lower than the latter and very likely similar to our threshold dose.
- In our protocol for inhalation tests, besides the physiologic endpoint, we have also included a clinical criterion— Ae presence of mild wheezing in “positive” inhalation tests. Although mild symptoms of asthma may not always be associated with mild wheezing, the presence of the latter gives more weight to the significance of a physiologic change which, whatever its magnitude, is essentially arbitrary. Others have previously shown that during inhalation tests with bronchoconstrictor agents, the first chest complaints appear when —A FEV1 is ~10 percent or slightly high-er. When this degree of physiologic impairment is associated with wheezing, as it was in our study, other physiologic changes suggesting the presence of bronchoconstriction are also present, eg, an increase in the alveolar slope of the single breath N2 washout, increase in the work of breathing and dynamic lung compliance and increase in the airway or pulmonary resistance.
- Acute or chronic decreases in FEVj of around 10 percent occurring in groups of subjects were found to be statistically significant.
- Recently, others have also used relatively mild attacks of asthma when such small airway responses were appropriate to the design of their study.
Study asthma inhalers in our category – https://onlineasthmainhalers.com/category/asthma-inhalers.
Nebulization was started with 0.005 mg histamine, 1 PNU allergen, and 0.025 mg acetylcholine [threshold doses (TD) of acetylcholine are frequently higher than TD of histamine]. The doses were doubled every 15 minutes (for drugs) or 30 minutes (for allergen). When chlorpheniramine or atropine were used to assess their influence on TDs, the drug or allergen challenge test proceeded as usual except that chlorpheniramine was injected 90 minutes, and atropine 30 minutes, before the expected therapeutic response (ie, immediately after nebulization of l/64th of TD of histamine or acetylcholine and &th of TD of allergen for chlorpheniramine; and immediately after the nebulization of Xth of TD of histamine or acetylcholine and X of TD of allergen for atropine).
The design of study included the following steps: (a) determination on separate days of TD of inhaled histamine and allergen; (b) repetition of the same protocol mentioned above in order to assess the reproducibility of these TDs. (This step led to the inclusion in the study of only nine out of the 15 initial subjects); (c) determination of TD of histamine and allergen after pretreatment with 10 mg chlorpheniramine administered intravenously. If chloipheniramine administered intravenously would protect against mild allergen-induced asthma, the study should attempt to determine whether this action is due to the antihistaminic or anticholinergic properties of this drug. For this purpose, in three subjects, the threshold response to inhaled acetylcholine was determined and checked for reproducibility. Then the TDs of acetylcholine were determined after pretreatment with chlorpheniramine, and on separate days, atropine sulfate subcutaneously. Atropine was used in a dose which had a dose ratio against acetylcholine identical with that of the previously used dose of chlorpheniramine. The TD of allergen was again determined, this time in the presence of atropine (http://www.news-medical.net/drugs/Atropine-Injection.aspx).
In order to determine the stability of TDs of allergen, in two subjects, after completion of allergen-stropine test, the TD of allergen was again assessed, without any drug pretreatment.
The challenge tests with drugs and allergens were performed two or three days apart, respectively. Steps (a) through (c) lasted 15 to 21 days, and the following steps lasted an additional 12 to 18 days. The subjects remained asymptomatic during this period of time.
Statistical Analysis
Student’s f-test for paired observations, the test applied by others for the analysis of dose responses based on arbitrary physiologic criteria, was used in this study to assess the significance of changes in threshold doses produced by chlorpheniramine and atropine. The study has been approved by the Committee on Human Investigation of the University of Chicago. Informed consent was obtained from each subject.
Figure 1. Dose response curves to inhaled histamine in six asthmatic subjects. Horizontal line (90 percent baseline FEV1) marks minimum decrease in FEV1 required for positive test Note reproducibility of histamine dose as determined with an endpoint characterized by —A FEV1 > 10%.